In recent years, neuroscience has gained a better understanding of the mechanisms and dynamics of Alzheimer’s disease. However, despite these advances, there are still some treatment options; Research is progressing slowly due to the complexity of the disease. However, a team of researchers may have some hope with ADDvac1, an experimental peptide vaccine targeting pathogenic protein. It has successfully passed Phase II clinical trials, showing that it is safe and effective, producing good immunity. However, in view of the sample size and the parameters of the trial, additional studies are needed to determine the true efficacy of this immunotherapy.
An experimental vaccine designed to protect against Alzheimer’s disease has crossed the final stage in human testing, researchers report in a new study. In the second phase of the clinical trial, the vaccine candidate AADvac1, developed by biotechnology company Axon Neuroscience, was shown to be well tolerated by patients and to produce a response against certain signs of the disease.
Safe and effective vaccine
Of the 193 patients with mild Alzheimer’s disease, 117 received AADvac1, the rest served as a control group and received placebo instead. In a randomized, double-blind, 24-month trial, participants who received AADvac1 received the peptide-based vaccine in 11 doses administered during the trial and showed elevated immunoglobulin G antibody responses.
Researchers believe that AADvac1 is effective against harmful aggregations of toe proteins in the brain, which is thought to be one of the main symptoms of the disease. In support of this, the latest trial showed that AADvac1 was linked to the slow build up of neurofilament light chain protein (NfL), indicating slower neurodegeneration compared with placebo-acquired patients.
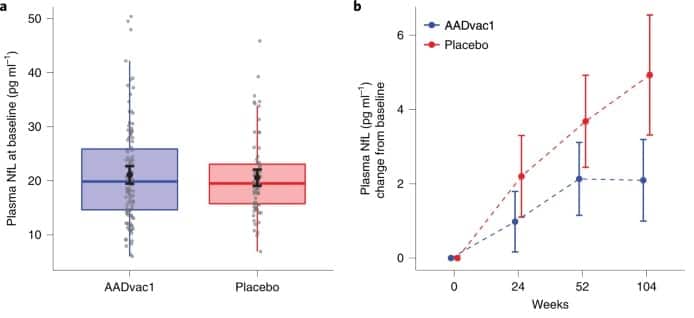
« To the best of my knowledge, tow-targeted immunotherapy has shown clear evidence of an effect on the neurodegenerative process and is a strong indication of clinical effect in patients with a confirmed biomarker profile of Alzheimer’s disease. », Explained Peter Novak, Principal Investigator in Clinical Research at Axon Neuroscience.
Limitations of Clinical Trial
While this disease-modifying effect is definitely a good development to look at, experimental treatment has not yet shown credible signs of significantly combating cognitive decline across the spectrum of patients with dementia. Alzheimer’s disease. ” No significant effect was found on the cognitive and functional tests on the overall study model Researchers write in their study.
It should be noted that these results came from a relatively small and early stage II clinical trial designed primarily to test the safety profile of an experimental vaccine. At this time, it was well tolerated and considered successful, showing no clinically significant adverse effects due to AADvac1.
On whether AADvac1 can deliver its promise of successfully treating cognitive decline, however, researchers say we need a bigger trial with more patients; And more patients with dementia, especially those associated with tau pathology. The small overall size of the current trial called ADAMANT and the lack of proportion of biomarker-based patient pathologies in the ensemble are the limitations we need to be aware of.
« This study was designed to identify only significant effects on clinical parameters, with 193 patients in the complete diagnostic set Researchers write that many patients in the study for diagnostic biomarkers did not meet certain criteria for toxin.
Action against tau protein
In other words, despite the ambiguity of some of the results, there are still positive signs here, especially when the analysis focuses on patients who have been linked to Alzheimer’s disease biomarkers, indicating that it is tau. Who is responsible for their condition, who will most likely respond to this vaccine.
In a specific subset of 109 patients tested positive for amyloid and tau biomarkers, estimates of their dementia rate and daily life activity indicated that treatment with AADvac1 could slow their decline. Cognitive and functional are up to 30% of the placebo group.
« These post-hack analyzes have significant limitations because they are not specified in the Clinical Study Protocol and are not corrected for multiplication tests. Therefore these results need to be carefully understood and confirmed in future clinical development. 2, Conclude authors.





More Stories
Healing Streams Live Healing Services with Pastor Chris: Miracles Await this March 14th – 16th, 2025!
Essential Care for Hermann’s Tortoise: A Guide to Thriving Pets
Nail Decisions: Which is Better for You, Acrylic or Gel?