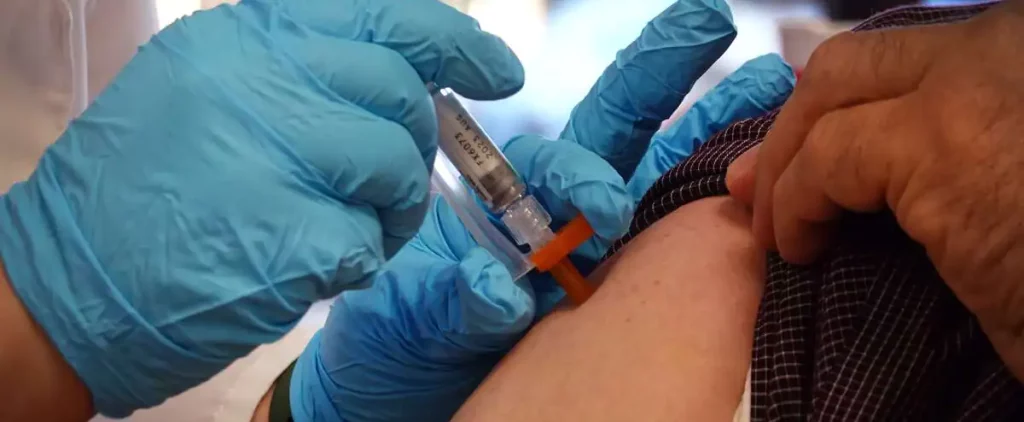
Health Canada has approved Pfizer-BioNTech’s updated COVID-19 Comirnaty vaccine that targets the BA.4 and BA.5 subvariants of Omicron and is approved for use as a booster dose in individuals 12 years of age and older.
• Also Read: The consequences of the epidemic among young people are studied
It is the second bivalent vaccine approved in the country, effective against both the early strain of Covid-19 and one of its variants, in this case, Omicron.
However, the first bivalent vaccine produced by Moderna attacked an early strain of the Omicron sub-variant. Pfizer’s vaccine completes the picture by attacking the more infectious Omicron BA.4 and BA.5 subvariants.
At a press briefing, federal Health Minister Jean-Yves Duclos urged Canadians to get their booster dose and “recharge their immunity” if it’s been more than six months since their last injection or their last infection with the virus.
“Two bivalent vaccines are now approved in Canada […] Both provide an excellent immune response and increased protection against the omicron variant,” he says.
“Health Canada licensed this vaccine after a thorough and independent scientific review of the evidence that demonstrated the safety and efficacy of this vaccine,” Health Canada said in a statement Friday.
Remember that Moderna’s bivalent vaccine has been available in Quebec since last month to protect against both of these variants.





More Stories
Saint Laurent Distillery went bankrupt
When even teachers buy peace with candies and curtains!
Trudeau: Bury of social cohesion