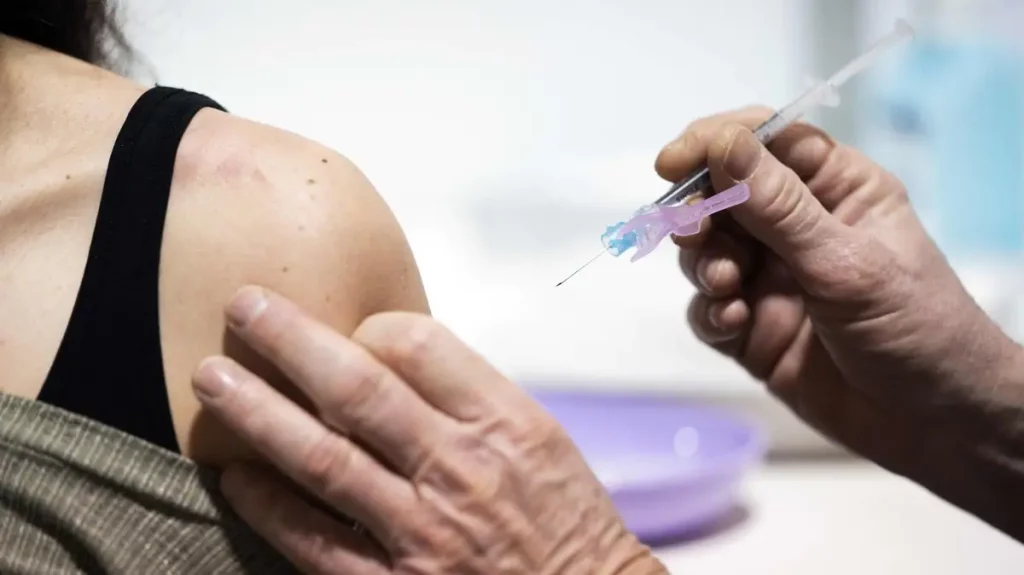
The U.S. Medicines Agency (FDA) on Friday expressed concern about the risk of myocarditis with novovax’s Kovid-19 vaccine, ahead of an expert meeting next week to determine the effectiveness of the remedy in the United States.
Also read: Kovid cases are on the rise again in Brazil
Also read: Normal termination of rapid test deliveries in December
Also read: Growth of variants: “There are worrying mutations”
The novovax vaccine is already approved in other countries, especially in Europe.
In the United States, an independent committee convened at the request of the FDA to evaluate data from Novavax’s clinical trials and make its recommendation will meet Tuesday. Upstream, the agency released a lengthy paper on Friday analyzing these results, as it has done for three other vaccines already authorized in the country.
In the wake of this the Novavox title collapsed 20% on the New York Stock Exchange.
According to the FDA, in trials conducted before the appearance of the omicron variant, the novovax vaccine was found to be 90% effective against symptomatic cases of the disease.
But six cases of myocarditis and myocardial infarction were found in the vaccinated group, as opposed to one case in the placebo group, the agency pointed out. Five cases were reported within two weeks of vaccination.
Of the 40,000 clinical trial participants, the identification of multiple cases potentially related to the vaccine, if there was a causal link, the risk of developing myocarditis after vaccination with NVX-CoV2373 may be higher than reported at the time. Messenger RNA vaccines are post-approval, “the FDA said in a statement.
The risk of myocarditis has been identified, especially in adolescents and adolescents after vaccination with Pfizer or Moderna. But during clinical trials of these vaccines, the FDA recalled that no cases had been found yet. She seems to be concerned that the risk with Novavox is high.
The company released a response statement on Friday: “We believe there is not enough evidence to establish a causal link between myocarditis cases and vaccines.” The population is ‘in any large enough database’ anyway.





More Stories
Ban this bad habit immediately!
Saint Laurent Distillery went bankrupt
When even teachers buy peace with candies and curtains!